Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 high energy resolution XAFS
high energy resolution XAFSYamamoto, Naoki*; Matsumura, Daiju; Hagihara, Yuto*; Tanaka, Kei*; Hasegawa, Yuta*; Ishii, Kenji*; Tanaka, Hirohisa*
Journal of Power Sources, 557, p.232508_1 - 232508_10, 2023/02
Times Cited Count:2 Percentile:29.01(Chemistry, Physical)Liu, B.*; Feng, R.*; Busch, M.*; Wang, S.*; Wu, H.*; Liu, P.*; Gu, J.*; Bahadoran, A.*; Matsumura, Daiju; Tsuji, Takuya; et al.
ACS Nano, 16(9), p.14121 - 14133, 2022/09
Times Cited Count:49 Percentile:98.33(Chemistry, Multidisciplinary)Kimata, Tetsuya*; Kakitani, Kenta*; Yamamoto, Shunya*; Shimoyama, Iwao; Matsumura, Daiju; Iwase, Akihiro*; Mao, W.*; Kobayashi, Tomohiro*; Yamaki, Tetsuya*; Terai, Takayuki*
Physical Review Materials (Internet), 6(3), p.035801_1 - 035801_7, 2022/03
Times Cited Count:7 Percentile:71.37(Materials Science, Multidisciplinary)Itoi, Hiroyuki*; Ninomiya, Takeru*; Hasegawa, Hideyuki*; Maki, Shintaro*; Sakakibara, Akihiro*; Suzuki, Ryutaro*; Kasai, Yuto*; Iwata, Hiroyuki*; Matsumura, Daiju; Owada, Mao*; et al.
Journal of Physical Chemistry C, 124(28), p.15205 - 15215, 2020/07
Times Cited Count:8 Percentile:40.15(Chemistry, Physical)Yasuda, Satoshi; Uchibori, Yosuke*; Wakeshima, Makoto*; Hinatsu, Yukio*; Ogawa, Hiroaki; Yano, Masahiro; Asaoka, Hidehito
RSC Advances (Internet), 8(66), p.37600 - 37605, 2018/11
Times Cited Count:12 Percentile:42.69(Chemistry, Multidisciplinary)We present a quantitative study on the effect of a newly obtained thermal history on the formation of Fe-N-C catalytic sites. A short and repeated heating process is employed as the new thermal history, where short heating (1 min) followed by quenching is applied to a sample with arbitrary repetition. Through electrochemical quantitative analysis, it is found that the new process effectively increases the Fe-N-C mass-based site density (MSD) to almost twice that achieved using a conventional continuous heating process, while the turn-over frequency (TOF) is independent of the process. Elemental analysis shows that the new process effectively suppresses the thermal desorption of Fe and N atoms during the initial formation stage and consequently contributes to an increase in the Fe-N-C site density. The resultant catalytic activity (gravimetric kinetic current density (0.8 V vs. RHE)) is 1.8 times higher than that achieved with the continuous heating process.
Hayashida, Kazuki; Kato, Toshihiro*; Kubota, Mitsuru*; Murakami, Hiroaki; Amano, Yuki; Iwatsuki, Teruki
Chikyu Kagaku, 52(1), p.55 - 71, 2018/03
In this study, the simulated experimental drift was constructed in the granite of 500 m depth at Mizunami Underground Research Laboratory, and the hydrochemical process after the drift closure was observed. The groundwater chemistry around the drift changed with the change of the groundwater flow in the fractures when the gallery was constructed. The redox potential increased due to the infiltration of oxygen from the drift into the rock. After closing the drift, the redox potential of the groundwater plunged due to microbial activity, while the groundwater became alkalized conditon due to the influence of cement material such as shotcrete. The amount of cement material consumed for this alkalization was small, and it was considered that its influence would last long in accordance with the amount of cement used.
Asazawa, Koichiro*; Kishi, Hirofumi*; Tanaka, Hirohisa*; Matsumura, Daiju; Tamura, Kazuhisa; Nishihata, Yasuo; Saputro, A. G.*; Nakanishi, Hiroshi*; Kasai, Hideaki*; Artyushkova, K.*; et al.
Journal of Physical Chemistry C, 118(44), p.25480 - 25486, 2014/11
Times Cited Count:15 Percentile:44.91(Chemistry, Physical)Taniguchi, Naoki; ; Kawasaki, Manabu*; Masugata, Tsuyoshi*
JNC TN8400 2001-001, 56 Pages, 2000/12
It is necessary to clear the effects of corrosion products on the corrosion life time of carbon steel overpack for geological isolation of high-level radioactive waste(HLW). Especially, it is important to understand the effects of magnetite because magnetite as a simulated corrosion product is reported to accelerate the corrosion rate of carbon steel. In this study, corrosion tests to reproduce the acceleration of corrosion due to magnetite was performed and the mechanism of the acceleration was investigated to evaluate the effects of magnetite as a corrosion product. Based on the results of experiments, following conclusions are obtained ; (1)Magnetite powder accelerates the corrosion rate of carbon steel. The main reaction of corrosion under the presence of magnetite is the reduction of Fe(III) in magnetite to Fe(II), but the reaction of hydrogen generation is also accelerated. The contribution of hydrogen generation reaction was estimated to be about 30% in the total corrosion reaction based on the experimental result of immersion test under the presence of magnetite. (2)Actual corrosion products containing magnetite generated by the corrosion of carbon steel protect the metal from the propagation of corrosion. The corrosion depth of carbon steel overpack due to magnetite was estimated to be about 1 mm based on the results of experiments. Even if the effect of magnetite is taken into the assessment of corrosion lifetime of overpack, total corrosion depth in 1000 years is estimated to be 33 mm, which is smaller than the corrosion allowance of 40 mm described in the second progress report on research and development for the geological disposal of HLM/ in Japan. It was concluded that the effect of magnetite on the corrosion life time of carbon steel overpack is negligible.
Iwase, Masanori*
JNC TJ8400 2000-063, 78 Pages, 2000/03
This study is aimed at controlling oxidation reaction of molten metal by ash in incineration systems, and at positively utilizing the oxidation reaction for decontamination of slag. In this year, in order to investigate physico-chemical properties of mixed fused salt containing alkali sulfates, with special focus on the behaviour of oxygen anion in the melts, Cu / Cu
/ Cu redox equilibrium experiments were carried out. Among the effect of various parameters on Cu
redox equilibrium experiments were carried out. Among the effect of various parameters on Cu / Cu
/ Cu ratio in binary and ternary alkali sulfate melts, the effect of partial pressures of oxygen and SO
ratio in binary and ternary alkali sulfate melts, the effect of partial pressures of oxygen and SO was mainly investigated in the study. Variation in Cu
was mainly investigated in the study. Variation in Cu / Cu
/ Cu ratio were presented as the function of partial pressures of oxygen and SO
ratio were presented as the function of partial pressures of oxygen and SO , respectively. Possible thermodynamic interpretation were made on the experimental results. In addition, the dissolution of Cr
, respectively. Possible thermodynamic interpretation were made on the experimental results. In addition, the dissolution of Cr O
O in mixed alkali sulfates were also investigated as a first step to elucidate the mechanism of hot corrosion. With this investigation, an important finding was obtained that the solubility of Cr
in mixed alkali sulfates were also investigated as a first step to elucidate the mechanism of hot corrosion. With this investigation, an important finding was obtained that the solubility of Cr O
O for melts with same average ionic radius, in other words, oxygen ion activity, were essentially identical under constant temperature and atmosphere.
for melts with same average ionic radius, in other words, oxygen ion activity, were essentially identical under constant temperature and atmosphere.
Miki, Takahito*; Sasamoto, Hiroshi; Chiba, Tamotsu*; Inagaki, Manabu*; Yui, Mikazu
JNC TN8400 2000-007, 32 Pages, 2000/01
This report presents a summary of literature survey about geochemical reactions which are important to evaluate the redox conditions in the near field rock mass and buffer. The results of literature survey are summarized as follows; (1)Minerals including ferrous iron and organic materials in the rock mass are important reductants. Initial stage after closure of repository, oxygen will be consumed by pyrite, because the reaction rate between pyrite and oxygen is relatively fast. (2)It is possible to estimate the redox capacity for reductants by rock (mineral)-water iteraction experiment in a laboratory. And it is expected that the ferrous iron-rich rock and higher porosity rock may have bigger redox capacity. (3)It is possible to estimate the oxygen consumption rate by reductants such as minerals including ferrous iron. The rate law and rate constant for the oxidation reaction of ferrous iron in the solution are also determined. As a conclusion, it seems that we can evaluate kinetically the evolution of geochemical conditions in the near field rock mass and buffer by excavation of drifts, based on data derived from these existing literatures.
Yamaguchi, Tetsuji
Genshiryoku Bakkuendo Kenkyu, 6(1), p.147 - 149, 1999/12
no abstracts in English
Oda, Chie; Arthur, R. C,*; Sasamoto, Hiroshi; Shibata, Masahiro; Yui, Mikazu; Neyama, Atsushi*
JNC TN8400 99-079, 287 Pages, 1999/09
Two thermodynamic databases for geochemical calculations supporting research and development on geological disposal concepts for high level radioactive waste are described in this report. One, SPRONS.JNC, is compatible with thermodynamic relations comprising the SUPCRT model and software, which permits calculation of the standard molal and partial molal thermodynamic properties of minerals, gases, aqueous species and reactions from 1 to 5000 bars and 0 to 1000 C. This database includes standard molal Gibbs free energies and enthalpies of formation, standard molal entropies and volumes, and Maier-Kelly heat capacity coefficients at the reference pressure (1 bar) and temperature (25
C. This database includes standard molal Gibbs free energies and enthalpies of formation, standard molal entropies and volumes, and Maier-Kelly heat capacity coefficients at the reference pressure (1 bar) and temperature (25 C) for 195 minerals and 16 gases. It also includes standard partial molal Gibbs free energies and enthalpies of formation, standard partial molal entropies, and Helgeson, Kirkham and Flowers (HKF) equation-of-state coefficients at the reference pressure and temperature for 1147 inorganic and organic aqueous ions and complexes. SPRONS.JNC extends similar databases described elsewhere by incorporating new and revised data published in the peer-reviewed literature since 1991. The other database, PHREEQE.JNC, is compatible with the PHREEQE series of geochemical modeling codes. It includes equilibrium constants at 25
C) for 195 minerals and 16 gases. It also includes standard partial molal Gibbs free energies and enthalpies of formation, standard partial molal entropies, and Helgeson, Kirkham and Flowers (HKF) equation-of-state coefficients at the reference pressure and temperature for 1147 inorganic and organic aqueous ions and complexes. SPRONS.JNC extends similar databases described elsewhere by incorporating new and revised data published in the peer-reviewed literature since 1991. The other database, PHREEQE.JNC, is compatible with the PHREEQE series of geochemical modeling codes. It includes equilibrium constants at 25 C and 1 bar for mineral-dissolution, gas-solubility, aqueous-association and oxidation-reduction reactions. Reaction enthalpies, or coefficients in an empirical log K(T) function, are also included in this database, which permits calculation of equilibrium constants between 0 and 100
C and 1 bar for mineral-dissolution, gas-solubility, aqueous-association and oxidation-reduction reactions. Reaction enthalpies, or coefficients in an empirical log K(T) function, are also included in this database, which permits calculation of equilibrium constants between 0 and 100 C at 1 bar. All equilibrium constants, reaction enthalpies, and logK(T) coefficients in PHREEQE.JNC are calculated usig SUPCRT and SPRONS.JNC, which ensures that these two databases are mutually consistent. They are also internally consistent insofar as all the data are compatible with basic thermodynamic definitions and functional relations in the SUPCRT ...
C at 1 bar. All equilibrium constants, reaction enthalpies, and logK(T) coefficients in PHREEQE.JNC are calculated usig SUPCRT and SPRONS.JNC, which ensures that these two databases are mutually consistent. They are also internally consistent insofar as all the data are compatible with basic thermodynamic definitions and functional relations in the SUPCRT ...
Kihara, Sorin*; Yoshida, Zenko; Aoyagi, Hisao; Maeda, Koji*; Shirai, Osamu; Kitatsuji, Yoshihiro; Yoshida, Yumi*
Pure and Applied Chemistry, 71(9), p.1771 - 1807, 1999/09
Times Cited Count:55 Percentile:83.61(Chemistry, Multidisciplinary)no abstracts in English

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO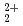 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Hashizume, Shuji; Matsumoto, Junko; Bamba, Tsunetaka
Genshiryoku Bakkuendo Kenkyu, 5(1), p.45 - 49, 1998/08
no abstracts in English
Tachimori, Shoichi; *
JAERI-Data/Code 96-030, 116 Pages, 1996/10
no abstracts in English
Yokoi, Koichi*; Yoneda, Yoshihiro*; Takahara, Hiroyuki*; Moriya, Toshifumi*
PNC TJ1380 96-002, 68 Pages, 1996/03
None
*; Nakamura, Hisashi; Kanazawa, Katsuo; Fujiki, Kazuo; *
Chuzo Kogaku, 68(8), p.644 - 649, 1996/00
no abstracts in English